Astrazeneca releases promising data on its coronavirus vaccine
By Rebecca Robbins and Benjamin Mueller
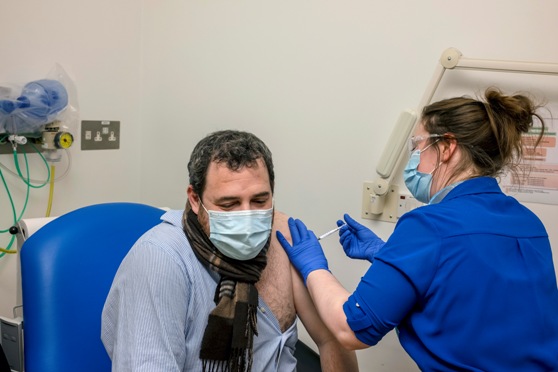
LONDON – AstraZeneca and the University of Oxford announced on Monday (23) that their inexpensive, easy-to-produce coronavirus vaccine appears effective, the latest in a string of encouraging results this month from vaccine developers.
An early analysis of data from late-stage clinical trials found that the vaccine was either 62% or 90% effective, depending on the manner in which the doses were given. On average, the vaccine was 70% effective, AstraZeneca said.
AstraZeneca’s announcement came on the heels of Pfizer and Moderna releasing trial results that showed their vaccines to both be about 95% effective. Unlike the Pfizer and Moderna vaccines, AstraZeneca’s offering can be stored for at least six months in standard refrigerators and is relatively easy and inexpensive to mass-produce.
AstraZeneca’s results could significantly strengthen the global effort to produce enough vaccine to start defusing the pandemic: The price of the shot, at $3 to $4, is a fraction of that of some other potential vaccines. AstraZeneca has pledged to make it available at cost around the world until at least July 2021 and in poorer countries in perpetuity.
AstraZeneca said it expected to begin distributing the vaccine this year and that it would be able to make up to 3 billion doses next year. That would be enough to inoculate nearly 1 in 5 humans worldwide.
The varying effectiveness of AstraZeneca’s vaccine reflected differences in how doses were administered in the late-stage trials. In the dosing plan that was 90% effective, study participants were given a half-dose of the vaccine and then, a month later, a full dose. The vaccine was less effective when people were given a standard full dose upfront, followed a month later by another full dose.
The analysis, which looked at data from participants in Britain and Brazil, did not turn up any serious safety issues that were confirmed to be related to the vaccine. It had come under global scrutiny after AstraZeneca temporarily paused its trials in September after a participant in Britain developed a neurological illness.
Oxford and AstraZeneca said they would submit the trial data to regulators in Britain, the European Union and Brazil and would seek emergency authorization to start distributing the vaccine in those places. British and European Union regulators have been conducting so-called rolling reviews of the vaccine, which could hasten the authorization process.
The path toward the vaccine being available in the United States is less clear. Because clinical trials in the United States were paused more than a month, longer than in Britain and some other countries, the US results are not expected until next year. And AstraZeneca has not been testing the more-effective half-dose regimen in its US trials. The company said it would work with the Food and Drug Administration to add it as quickly as possible to its ongoing trial.
In the meantime, AstraZeneca said, it will share its latest British and Brazilian trial data with the FDA this week. The company is seeking guidance on whether it should formally submit the findings for review and authorization for emergency use even though the US trial remains underway.
Pfizer and Moderna’s vaccines are based on similar messenger RNA technology. It uses synthetic genetic material to stimulate cells to produce a harmless viral protein that the immune system can learn to attack.
The Oxford-AstraZeneca vaccine is different. It uses a weakened version of a chimpanzee adenovirus as a delivery vehicle to ferry coronavirus genes into human cells. That trains the immune system to fight future attacks from the actual coronavirus.
The company said its early analysis was based on 131 symptomatic coronavirus cases that turned up in participants at least two weeks after they had received their second shot.
None of the vaccinated people who developed the disease required hospitalization, AstraZeneca and Oxford said.
“Today marks an important milestone in our fight against the pandemic,” AstraZeneca’s chief executive, Pascal Soriot, said in a statement. “This vaccine’s efficacy and safety confirm that it will be highly effective against COVID-19 and will have an immediate impact on this public health emergency.”
Dr. Saad B. Omer, the director of the Yale Institute for Global Health, pointed to several potential explanations for why the dosing regimens yielded different results. There could be key differences between the two groups, such as participants’ ages or their past exposure to similar viruses that influenced how they responded.
The design of the vaccine could also play a role: The full dose could be giving participants immunity to the delivery vehicle and dampening their subsequent immune response. Sample size may also be at play. Fewer participants received the smaller first dose, giving their results less statistical power.
Omer cautioned against drawing definitive conclusions until more details were available. “I would withhold my judgment until we specifically look at more data,” he said.
Pam Cheng, an executive vice president at AstraZeneca, told reporters on Monday that if the company received regulatory approval, it planned to have 4 million doses available in Britain by the end of the year. By the end of March, the company said, it would have at least 300 million doses of finished vaccine ready to distribute globally.
Even without delays, however, the vaccine is still a long way from being widely available. Regulators must assess the study data and decide whether to authorize the vaccine. AstraZeneca must ramp up production and work with government officials to roll out doses. And in the first weeks and months after the vaccine is authorized, it is expected to be available only to the highest-priority groups, likely health workers first, followed by other vulnerable groups.
-New York Times